

Explain how the volume of the bubbles exhausted by a scuba diver change as they rise to the surface, assuming that they.
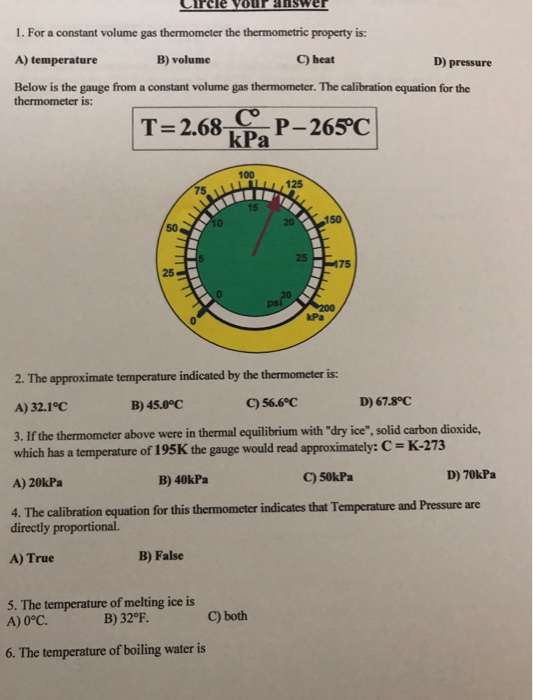
The volume of the tire can only expand so much before the rubber gives and releases the build up of pressure. The constant volume gas thermometer plays a crucial role in. Note: There are different types of thermometer present for different purposes, like- clinical thermometer laboratory thermometer etc. As temperature of a gas increases, pressure will also increase based on the ideal gas law. Plots of pressure vs temperature for three different gas samples extrapolate to absolute zero. A clinical mercury thermometer has mercury in them which expands with the temperature.

A clinical thermometer is a small medical thermometer with a short but finely calibrated range. Some means of converting this change into a numerical this change into a numerical value.Ģ. A temperature sensor- bulb of mercury-in-glass thermometer acts as a temperature sensor in this some changes occur with a change in temperature. The additional condition imposed by the problem may be expressed analytically. A thermometer has two important elements that are:ġ. The constant-volume gas thermometer, in which the pressure of the gas. The more is the friction in gases the more is its viscosity.įurthermore, a thermometer is a device that measures temperature or temperature gradient. When the temperature is increased the particles of gas move faster and collide more often, resulting in greater resistance of friction. Gas Thermometer 1/20/17 Abstract: This experiment involved using a constant pressure (isobaric) gas thermometer, and a constant volume (isochoric) gas. a constant pressure process is said to be isobaric. This relationship is known as Charles law or Gay-Lussacs law. The ratio of volume to temperature is constant when pressure is constant. Therefore, we get the required temperature of the bath at the given height and temperature, which is given by the above result.Īdditional information: we know that gases are made up of particles that are very far apart. In gas thermometers, the pressure rise connected with a temperature increase is used for measuring the temperature In gas filled temperature gauges (also called gas thermometers or gas-in-metal thermometers), a gas is used as the thermometric fluid instead of an liquid as it is in liquid-in-metal thermometers. volume-temperature (constant pressure) The volume of a gas is directly proportional to its temperature when pressure is constant. Also, we will study some basics of a thermometer and temperature. In this lecture, we will be analyzing the behavior of gases in the pressure and temperature range corresponding to heat engines, and in this range the Ideal. Further, we will get the result by substituting the given values to the expression. Hint: In this question, we will use the relation between the temperature change and the height change or the mercury reading change. where p is gas pressure, V is volume, mu is the number of moles, R is the universal gas constant ( 8.3144 j/(oK mole)), and T is the absolute temperature.


 0 kommentar(er)
0 kommentar(er)
